Looking back at my time before graduate school at University of Michigan, no one would believe that I am now this far in my academic career and fully involved in chemistry research. In 2011, I was diagnosed with an eye tumor in my left eye socket right before my first semester of the Ph.D. program at the University of Michigan, Ann Arbor. It was shocking since I had always been healthy and never had any major physical problems. The diagnosis on my eye tumor was made through an MRI scan, which really intrigued me to imaging techniques for the first time. Interestingly enough, at University of Michigan, I was fortunately given an opportunity to conduct a collaborative research to translate transition metal catalyzed fluorination methods into radiofluorination for tracers of positron emission tomography (PET). I was glad to be doing a research that deals with improving imaging techniques that helped me receive an accurate diagnosis for treatment. This collaboration has been really eye-opening and integrated me to really think toward developing not only conceptually interesting, but also practical fluorination means using organometallic reagent.
I am highly honored to receive the prestigious Howard Hughes Medical Institute International Student Research Fellowship that allows me to continue on my Ph. D. study without financial concern as well as continue collaborating with the PET facilities to pursue my research goals. It really helped me contemplate the big picture and how my research could help the community. The fellowship application process was quite stressful, as I had to be invited to apply through a departmental nomination and nomination by Rackham. I spent nearly a month completing the final application to the HHMI, asking for feedback from my advisor, labmates and even non-chemistry major friends so that my language was comprehensive to referees from different academic disciplines. Applying for this fellowship was a great experience and gave me more confidence and courage to apply for postdoctoral fellowships in the future. For the remaining time at U-M, I would love to pursue my goals to develop a synthetic procedure that can be widely utilized to make important organofluorine compounds.
In the Sanford lab, we are interested in developing a synthetic approach for a challenging carbon-fluorine bond formation by using a transition metal catalyst (Figure 1). In industry or PET community, we still relied on classical methods to produce organofluorine compounds that typically require harsh conditions to accomplish such energy demanding transformations. However, transition metal catalysis allows a lower energy barrier associated with such bond formation and therefore is widely utilized as an important synthetic tool to mitigate a number of difficult chemical processes today.
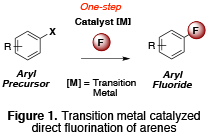
Figure1: Transition metal catalyzed direct fluorination of arenes.
Currently, my research is focused toward developing a new means to synthesize a small complex tracer labeled with a radionuclide fluorine-18 (18F) for positron emission tomography. Positron emission tomography (PET) is a minimally invasive imaging technique that provides kinetic physiochemical information. A PET scan can measure such vital functions as blood flow, oxygen use, and glucose metabolism, which helps doctors identify abnormal from normal functioning organs and tissues. The scan can also be used to evaluate the effectiveness of a patient’s treatment plan, allowing the course of care to be adjusted if necessary. Currently, PET scans are most commonly used to detect cancer, heart problems (such as coronary artery disease and damage to the heart following a heart attack), brain disorders (including brain tumors, memory disorders, seizures) and other central nervous system disorders. While CT and MRI detect changes a little later as the disease proceeds to cause changes in the structure of organs or tissue, a PET scan can often detect these very early changes. This imaging technique garnered attentions particularly for a potential that it may detect deficiencies in insidious, microscopic protein that has been found in the brain tissue of patients after death. It may now be detectable in living people by scanning their brains.
The current major problem in the PET community is that the type of PET scan they use is really not specific to what we’re looking at, partly due to there are not practical, good means to make such a tracer of interest.
For instance, currently in the U.S. 90% of PET scans are conducted with 18F-FDG ([18F]-Fluorodeoxyglucose). 18F-FDG can image a glucose metabolism in the human body; therefore, an excellent imaging agent to diagnose and monitor the post treatment of cancers. Despite technological developments coupled with the appearance of these radiopharmaceuticals have firmly established PET, there are still significant limitations. For example, 18F-FDG has diagnostic limitations due to the high glucose uptake by normal brain tissue. Figure 2 shows a brain image of a patient undergoing evaluation for brain tumors (Jacob et al. Ind. J. Nuc. Med. 2011, 26, 139). The left image was created by 18F-FDG where as the right image shows a PET scan by an amino acid 18F-L-DOPA. Clearly seen from the image, 18F-L-DOPA is suited for imaging of brain tumors because o the low uptake in normal brain tissue, other than corpus striatum neurons. Classical neucleophlic methods work for such electron-rich substrates (organic molecules with high electron density) require very harsh conditions for rapid fluorine incorporation. Overall, there is no reliable method for straightforward synthesis of complex small tracers for PET.
I was fortunate to collaborate with Dr. Peter J.H. Scott, a research investigator at University of Michigan, Ann Arbor. Recently, I was able to develop a mild [18F]fluorination condition using an inexpensive transition metal catalyst Copper (Cu) (Ichiishi, N.; Sanford, M.S.; Scott, P. J. H. et. al Org. Lett., 2014, 16, 3224). As a preliminary result, we have evaluated a range of electronically different molecules. Under a single set of mild reaction condition we were pleased to find that our protocol affords good to excellent radiochemical conversions of electron-rich arenes, which were previously inaccessible using classical nucleophilic [18F]fluorination methods. Now we are actively working towards automation of the synthesis as well as improving the reaction condition. Ultimately, our goal is to translate our methodology to synthesize important radiotracers for clinical dose, including [18F]-L-DOPA. Excitingly, I am collaborating with the Raffel lab in order to apply this methodology to synthesize more clinically relevant molecules.
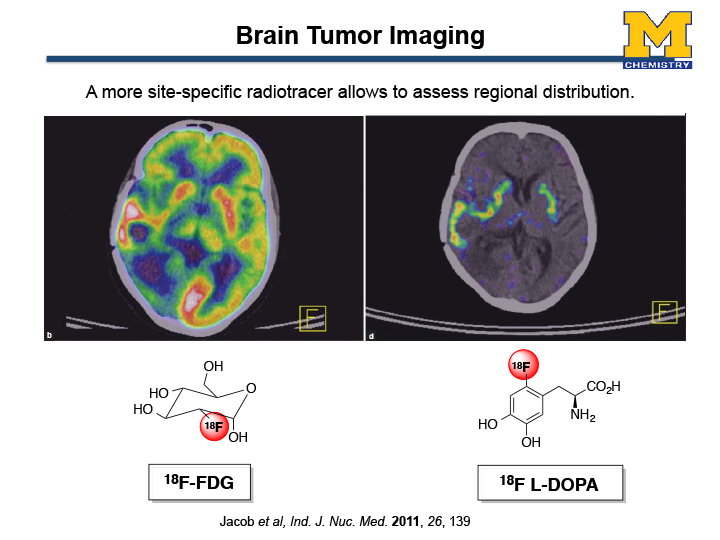
Figure 2. PET scan images of a patient with a brain tumor (a) an image by 18F-FDG; (b) an image by 18F L-DOPA.
